Background: Venetoclax is an oral small-molecule BCL-2 inhibitor, used in combination with rituximab (V+R) or as a monotherapy in adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL). Venetoclax in combination with obinutuzumab (V+G) for a fixed duration was recently approved by the European Medicines Agency for the management of previously untreated patients with CLL. With multiple additional therapies available for CLL including novel agents such as ibrutinib, Idelalisib + R (Ide+R) and acalabrutinib +/-G, treatment sequences that present the most favorable economic profiles remain unknown.
Objective: To estimate the total cumulative costs per patient of different treatment sequences for adult patients with CLL, and to evaluate the budget impact of introducing sequences with V+G in the first line in France.
Methods: A partitioned survival model was developed to assess the outcomes of treatment sequences commonly used in France up to three lines of therapy in CLL patients. The target population was previously untreated adult patients with CLL and ineligible for full-dose fludarabine treatment, with or without 17p deletion. The model adopted a French payer perspective with a 10-year time horizon. Patients were distributed into four states in each year: first line, second line, third line therapy, and death. The distribution of patients in each state were determined based on progression-free survival (PFS) and overall survival (OS) estimates for the first two lines of therapy reported in pivotal clinical trials. Other model inputs included treatment costs, monitoring and terminal care costs, transportation costs, tumor lysis syndrome (TLS) prevention costs for venetoclax-based therapies, and costs associated with adverse events (AEs). Additional inputs for the budget impact analysis (BIA) included published epidemiological estimates for patient numbers and proportions of patients receiving each treatment before and after introduction of first-line V+G in the treatment sequence. Patients were treated until progression or maximum duration specified on the label. PFS and OS from trials were extrapolated using an exponential distribution. Model outputs included total cumulative costs per patient, stratified by cost component for each treatment sequence. Results from the BIA included the total budget impact comparing before and after introduction of first line treatment with V+G in France.
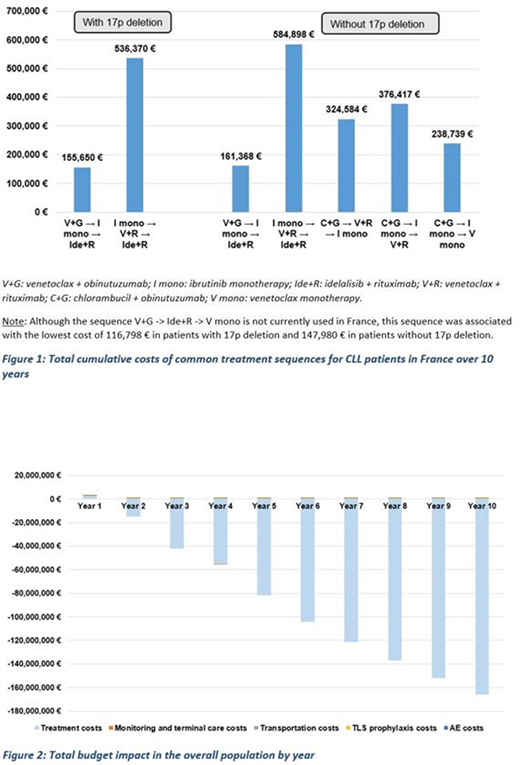
Results: In patients with 17p deletion, the treatment sequence with V+G as first line therapy was associated with lower costs compared to the sequence starting with I mono (Figure 1). Over a ten-year time horizon, the total cumulative costs per patient for V+G -> I mono -> Ide+R were 155,650 €, while they were 536,370 € for I mono -> V+R -> Ide+R. In patients without 17p deletion, the treatment sequence starting with V+G was the least costly option compared to sequences starting with ibrutinib or C+G (Figure 1). From a French national perspective, 238 patients with and 1,073 patients without 17p deletion were eligible for sequences starting with V+G. In terms of the budget impact of sequences with V+G in the first line, costs increased in the first year followed by declining costs in years 2 to 10. Total budget impact was cost saving at -860,046,119 € over 10 years (Figure 2).
Conclusions: Treatment sequences with fixed treatment combination of V+G in the first line led to the lowest total cumulative costs among sequences commonly used in France including those starting with innovative agents administered until disease progression for adult CLL patients ineligible for full-dose fludarabine. The budget impact of sequences with V+G in first line, regardless of 17p deletion status, is expected to be cost saving due to substantial reductions in treatment costs.
Fang:Analysis Group, Inc.: Current Employment, Other: Analysis Group, Inc. has received consulting fees from AbbVie for this study. Ravonimbola:AbbVie Inc.: Current Employment, Other: may own stocks and/or options of the company. Hazra:Analysis Group, Inc.: Current Employment, Other: Analysis Group, Inc. has received consulting fees from AbbVie for this study. Zhou:Analysis Group, Inc.: Current Employment, Other: Analysis Group, Inc. has received consulting fees from AbbVie for this study. Manzoor:Abbvie: Current Employment, Other: may hold stock or stock options. Levy:Janssen: Consultancy; AbbVie: Consultancy; Roche: Consultancy. Ysebaert:Roche: Consultancy; Janssen: Consultancy; AbbVie: Consultancy. Kabore:AbbVie Inc.: Current Employment, Other: may own stocks and/or options of the company. Sail:AbbVie: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal